The Value of Support Groups
Dorothy Leone-Glasser, RN, HHC, CEO Advocates for Responsible Care,
Chair Georgia Bio Patient Advocacy Alliance
When I was diagnosed with systemic lupus erythematosus at 19 years, I discovered a major void in the treatment of chronic illness –support counseling to help patients understand and cope with the challenges of their disease. To bridge this gap in my own practice as a Nurse Specialist, I took a 3-year internship in Chronic Illness Counseling at Columbia University, NYC. I was determined to fill that void so I created, a national award winning, Coping with Chronic Illness Project. The major component was providing support groups for those with chronic and terminal illness and their caregivers. The groups offered information and practical life-style engineering methods to address the physical and psychological aspects of illness, coping methods, pain and stress management strategies and communication and relationship skills with navigation approaches to our complex healthcare system.
Today we are partnering with GA Bio-Center for Global Health Innovation (CGHI) to focus on the science of patient input for medical research and development with consideration of downstream regulatory and legislative policy decision making. In doing so, we can explore gaps in knowledge and other barriers that impede progress, and discuss the value of patient input for research that addresses gaps and barriers that could help move the patient and Bio community forward.
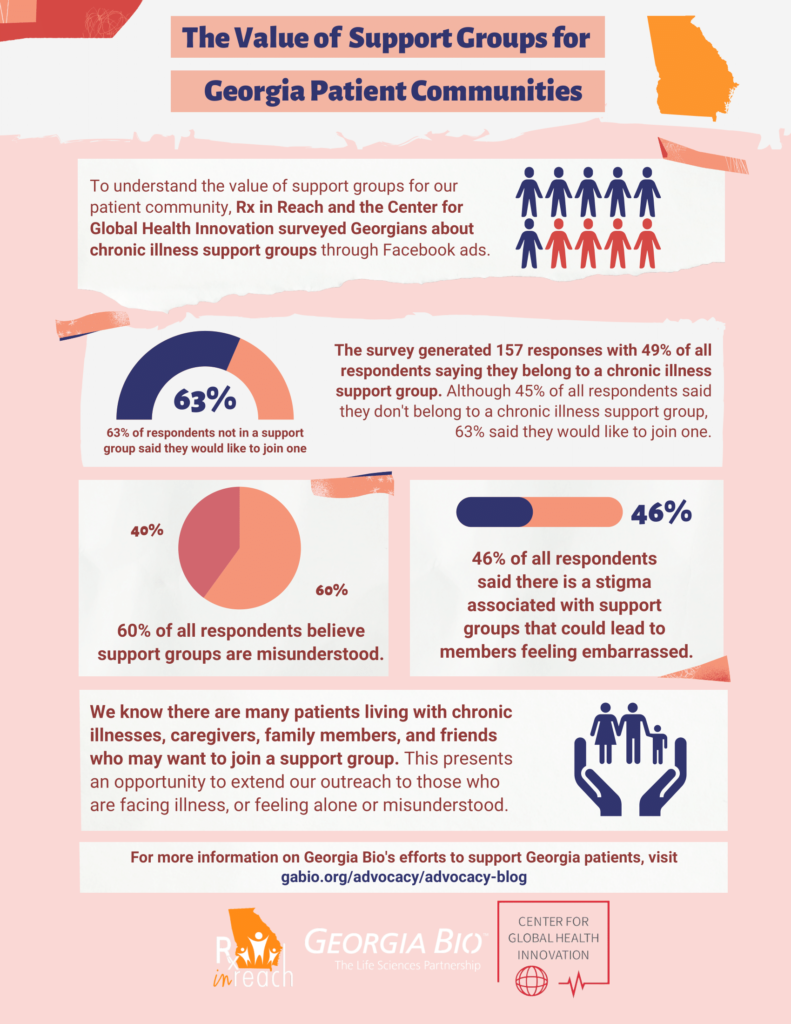
To understand the value of support groups on our patient community, Rx in Reach and CGHI launched a digital campaign to collect survey responses from Georgians about chronic illness support groups through Facebook Ads.
The survey generated 157 responses with 49% of all responders saying they belonged to a chronic illness support group. Although, 45% of all responders said they don’t belong to a support group for chronic illness; 63% of all responders said they would like to join a support group.
Other interesting findings from our survey were:
- 60% believe support groups are misunderstood
- 46% said there a stigma associated with support groups that could lead to members feeling embarrassed
- Responders stated they joined a support group because of
- feeling isolated
- needing help managing illness
- needing someone to listen
- their physician suggested it
Our findings show there is a strong stigma associated with seeking help from a support group. Support group members believe the public does not understand the need to connect with others who understand their daily challenges of living with chronic illness. Professional facilitated support groups offer a safe, non-judging environment for people to gain valuable information about disease processes, managing illness symptoms and healthcare system barriers while providing worthwhile life-style engineering to better cope with the rollercoaster of emotions and daily living complications. Survey responders, 20%, stated, their support group experience helped them, especially to feel less isolated and 15% of responders stated their group offered them the opportunity to gain new friends who understand their illness journey.
Summary
We know there are many with chronic illness, their caregivers, family members and friends who would want the chance to join a support group. This presents an opportunity for us to extend our outreach to those who are facing illness feeling alone and misunderstood so we can ensure their voices are heard with aid and encouragement offered to help them live their best life despite being ill. The work of this Patient Advocacy Alliance will continue to bring together our Bio community, patient support group members, advocate and medical organizations to improve health equity throughout our healthcare delivery system and clinical trials, while sharing strategy and actions that address important health policy issues.
Panelists

Kelly Barta
Coalition of Skin Diseases, President
After experiencing a prolonged and severe health crisis due to long-term use of topical steroids for eczema, Kelly became a passionate voice for those who were suffering. She was determined to bring change to the way eczema was understood and treated. Her advocacy efforts have led her to serve on several national dermatology nonprofits, bringing awareness to the great needs of this community and supporting those living with eczema and going through topical steroid withdrawal syndrome.
Kelly currently serves as President and CEO of the Coalition of Skin Diseases, an umbrella organization of more than 25 national skin-related patient groups, which exists to raise awareness of skin diseases and advocate for the 84+ million Americans living with skin conditions.
She has served as past president of the International Topical Steroid Awareness Network (ITSAN), and continues to support their mission as member of the Board of Directors. Additionally, Kelly sits on the Patient Advocacy Task Force of the American Academy of Dermatology and the Atopic Dermatitis Advisory Panel for Globalskin. She has presented on eczema and TSW to the FDA as well as before patient, academic and political audiences. Kelly is the recent author of her story, To Eczema With Love, https://toeczemawithlove.com/.
Kelly resides in Atlanta, Georgia with her two sons and enjoys taking in the great outdoors, whether it be the mountains, oceans, a walk in the woods or digging in her own garden.

Barbara Fleeman
WomenHeart Champion; Support Group Facilitator
Barbara grew up in Atlanta and moved back 6 years ago after spending 40 years building a successful career in entertainment consumer marketing/promotion. She was always very active in her personal and professional life. In 2013 she was diagnosed with severe pericarditis. Over the following 18 months. She continued to experience increasingly difficult episodes of shortness of breath, chest pain, fatigue, nausea, dizziness, and chest pressure. She went to see several doctors and was told that there was nothing wrong with her heart, even though she told them “My heart hurts”. She continued to become sicker. After a long course of indecision by medical professionals, she found help and a correct diagnosis of INOCA and Coronary Microvascular Dysfunction at the Barbra Streisand Women’s Heart Center at Cedars Sinai Medical Center in Los Angeles.
In 2015 she was invited to attend the WomenHeart Science and Leadership Symposium at the Mayo Clinic in Rochester, MN. After an intensive five-day event, she was trained to become a WomenHeart Champion. Barbara continues to share her experience with women to help educate them to the unique signs & symptoms of women’s heart disease, as well as, provide patient advocate support to women who are living with or at risk of heart disease.

J. Christopher Reed
Lupus Foundation of America-Georgia
Support Group Facilitator, Advocacy Chair
Christopher was diagnosed with lupus in 1990 at the age of 16. Lupus attacked his heart, lungs, kidneys, digestive system, veins, and nervous system. Christopher’s lupus journey began with chronic headaches and frequent muscle spasms then progressed to frequent fevers, arthritis, and tremendous weight loss. He was barely able wrap his hands around the steering wheels during driver’s ed class and walking up the stairwell to his bedroom felt like he was having a heart attack. In 2004, he developed stage 3 kidney disease. With his first job out of law school, Christopher was juggling kidney disease and horrible chemotherapy treatments. In 2018, he almost lost his life due to septic shock.
Overtime, Christopher continued to suffer from severe organ involvement. He was constantly worried about a lack of insurance and the cost of his life-saving medication.
Despite these serious lupus attacks, and moving back to his parent’s home, Christopher earned a bachelor’s degree with honors from the Georgia State University and a law degree from Tulane University. He attributes his success to God, the support of family and the support received at an early age from the Lupus Foundation of America, Georgia Chapter (LFAGA).
In speaking with Christopher, one can tell he is an avid advocate passionate about tackling healthcare policy and increasing lupus research. He previously chaired the Georgia Council on Lupus Education and Awareness (Council), a sponsored entity created by the state legislature to improve the lives of Georgians living with lupus. He has worked to establish the Georgia Lupus Collaborative, an advisory group and think tank charged with improving the lives of people with lupus in Georgia.
Christopher currently serves as a Support Group Facilitator, Advocacy Chair, and Project Manager for the Lupus Foundation of America-Georgia Chapter. His support group is specifically designed for men. As Advocacy Chair, he gives presentations on patient advocacy and public health policy as well as being a writer on Lupus. Each year, over a one hundred lupus advocates engage in Lupus Day at the Georgia State Capitol. He remains involved in Health Research and serves to increase African American participation in clinical trials through a series of education sessions, open discussion, and dialogues with African American physicians and African American researchers.
Christopher has been honored to be a recipient of several awards including the Mary Cann Achievement Award from the Lupus Foundation of America, Georgia Chapter.
The post The Value of Support Groups appeared first on Georgia Bio.